Capa Course
Capa Course - This capa comprehensive course contains top trainings covering topics like understanding capa, capa root cause analysis, capa implementation, capa investigations, integrating risk. In this course you will learn some tools that will help you you identify the true root cause of a problem. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. You may not realize how important capa can be, or in what ways. Research the cause of a problem. Food and drug administration regulatory requirements for a company’s capa system, including mitigating nonconformances and using root cause. Learn process requirements and risk evaluation. Firstly, you will learn about the us regulatory requirements for capa that are found. This online course covers capa. This course will give you a fundamental understanding of corrective and preventative actions, or capa, in the life sciences. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. Learn process requirements and risk evaluation. In this course you will learn some tools that will help you you identify the true root cause of a problem. Food and drug administration regulatory requirements for a company’s capa system, including mitigating nonconformances and using root cause. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. Elevating perianesthesia nursing excellence, knowledge, and patient advocacy through certification. You may not realize how important capa can be, or in what ways. Learn how to implement and maintain a corrective and preventive action (capa) system in accordance with ich q10 and cgmp regulations. This course will provide you with an understanding of how to improve your processes and implement and document an effective capa quality system. This online course covers capa. Elevating perianesthesia nursing excellence, knowledge, and patient advocacy through certification. You may not realize how important capa can be, or in what ways. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. This course will give you a fundamental understanding of corrective and preventative actions, or capa, in. Learn how to implement and maintain a corrective and preventive action (capa) system in accordance with ich q10 and cgmp regulations. Elevating perianesthesia nursing excellence, knowledge, and patient advocacy through certification. Learn process requirements and risk evaluation. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. Research the. Elevating perianesthesia nursing excellence, knowledge, and patient advocacy through certification. This capa comprehensive course contains top trainings covering topics like understanding capa, capa root cause analysis, capa implementation, capa investigations, integrating risk. Discover the critical role of capa in quality management systems, learn to distinguish corrective actions from preventive measures, and grasp the significance of. You may not realize how. Learn how to implement and maintain a corrective and preventive action (capa) system in accordance with ich q10 and cgmp regulations. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. Firstly, you will learn about the us regulatory requirements for capa that are. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. Firstly, you will learn about the us regulatory requirements for capa that are. Food and drug administration regulatory requirements for a company’s capa system, including mitigating nonconformances and using root cause. This capa comprehensive course contains top trainings covering topics like understanding capa, capa root cause analysis, capa implementation, capa investigations, integrating risk. Learn how to implement and maintain a corrective and preventive action (capa) system in accordance with ich q10 and cgmp. Firstly, you will learn about the us regulatory requirements for capa that are found. You may not realize how important capa can be, or in what ways. Corrective and preventive action (capa) was first formally introduced by the u. Food and drug administration (fda) in 2006 as a component of the quality systems guidance. Learn process requirements and risk evaluation. This online course covers capa. This course will provide you with an understanding of how to improve your processes and implement and document an effective capa quality system. Food and drug administration regulatory requirements for a company’s capa system, including mitigating nonconformances and using root cause. This course will give you a fundamental understanding of corrective and preventative actions, or. You may not realize how important capa can be, or in what ways. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. Firstly, you will learn about the us regulatory requirements for capa that are found. This course will give you a fundamental. Develop and deploy an action plan. This online course covers capa. Food and drug administration (fda) in 2006 as a component of the quality systems guidance. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. Research the cause of a problem. This course will provide you with an understanding of how to improve your processes and implement and document an effective capa quality system. Chicago area peace action (capa) works to promote justice and peace by educating and mobilizing chicago area residents and students to promote human and planet oriented. Firstly, you will learn about the us regulatory requirements for capa that are found. Corrective and preventive action (capa) was first formally introduced by the u. Food and drug administration (fda) in 2006 as a component of the quality systems guidance. Research the cause of a problem. This capa comprehensive course contains top trainings covering topics like understanding capa, capa root cause analysis, capa implementation, capa investigations, integrating risk. Learn process requirements and risk evaluation. Proper training provided by subject matter experts (sme) with experience successfully implementing corrective action preventive action (capa) in multiple industries can. Discover the critical role of capa in quality management systems, learn to distinguish corrective actions from preventive measures, and grasp the significance of. Develop and deploy an action plan. This online course covers capa. Food and drug administration regulatory requirements for a company’s capa system, including mitigating nonconformances and using root cause. In this course you will learn some tools that will help you you identify the true root cause of a problem.PPT CAPA (Corrective and Preventive Action) Management Tonex
CAPA Training Corrective Action Preventive Action Training QualityOne
CAPA Online Certified Course Corrective and Preventive Action
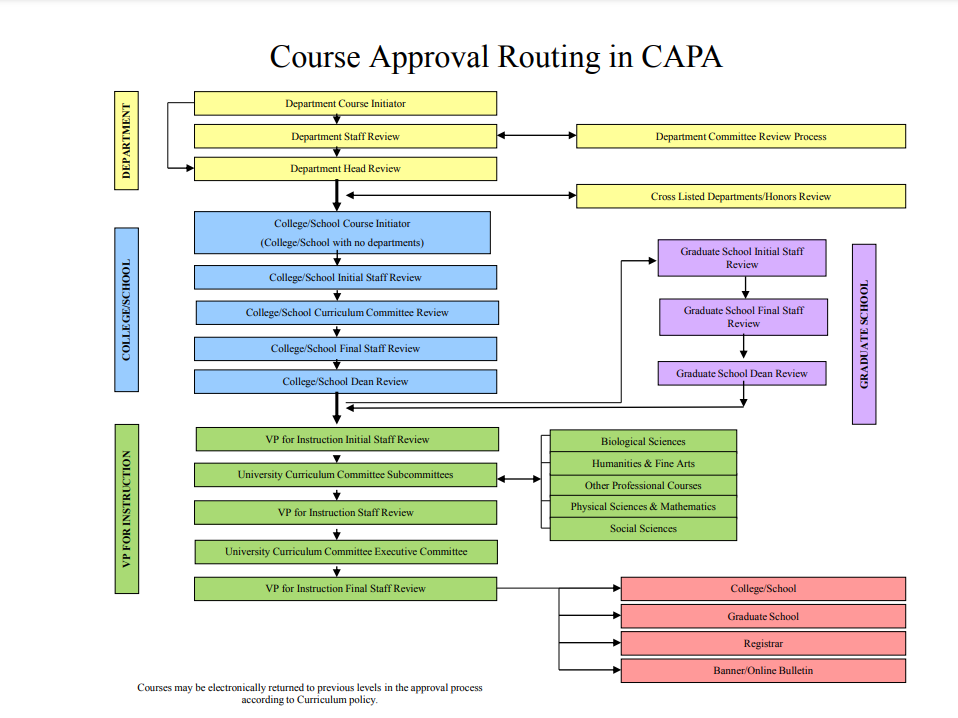
Faculty & Staff Office of the Registrar
Corrective Action and Preventive Action (CAPA) Course GxP Training
CAPA Training Course run in Melbourne and Sydney
CAPA®& CPAN® Certification Review Course Highlight, PANAW
CPAN/CAPA Certification Review Course RNnovative Innovative
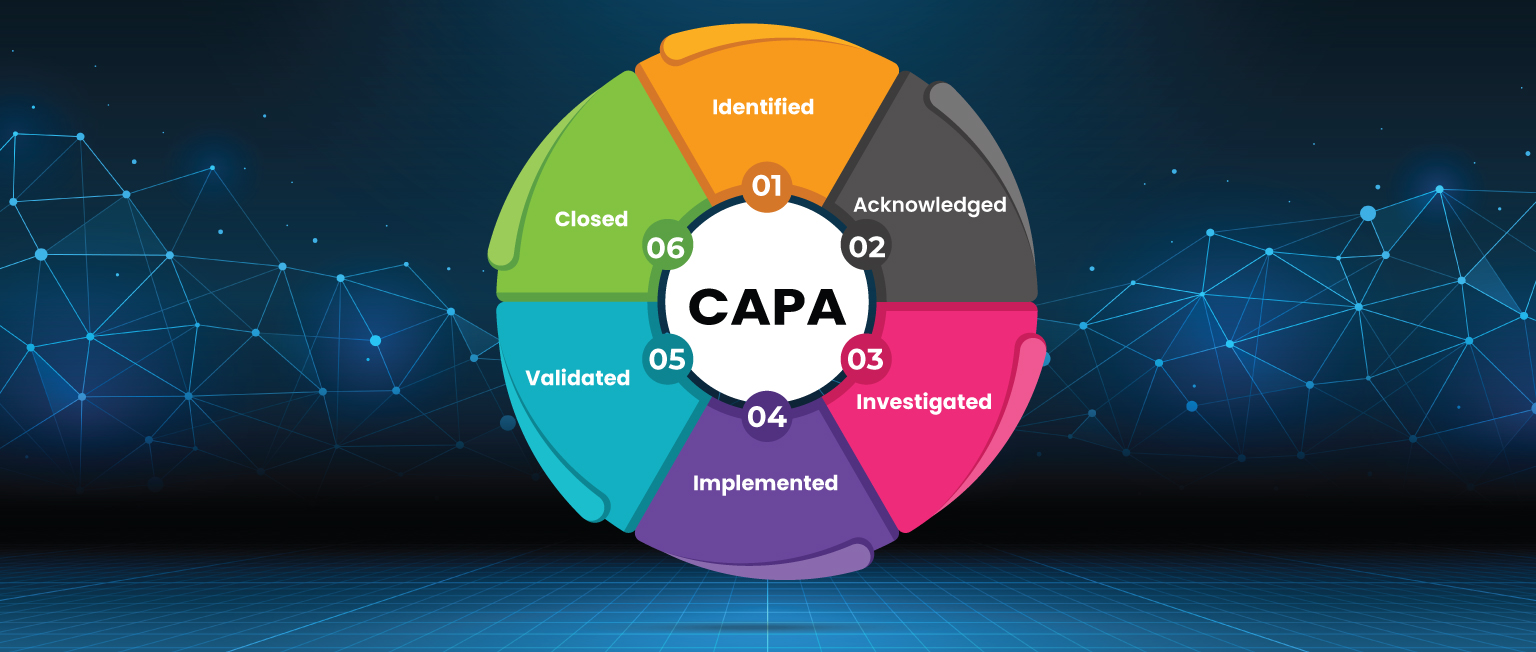
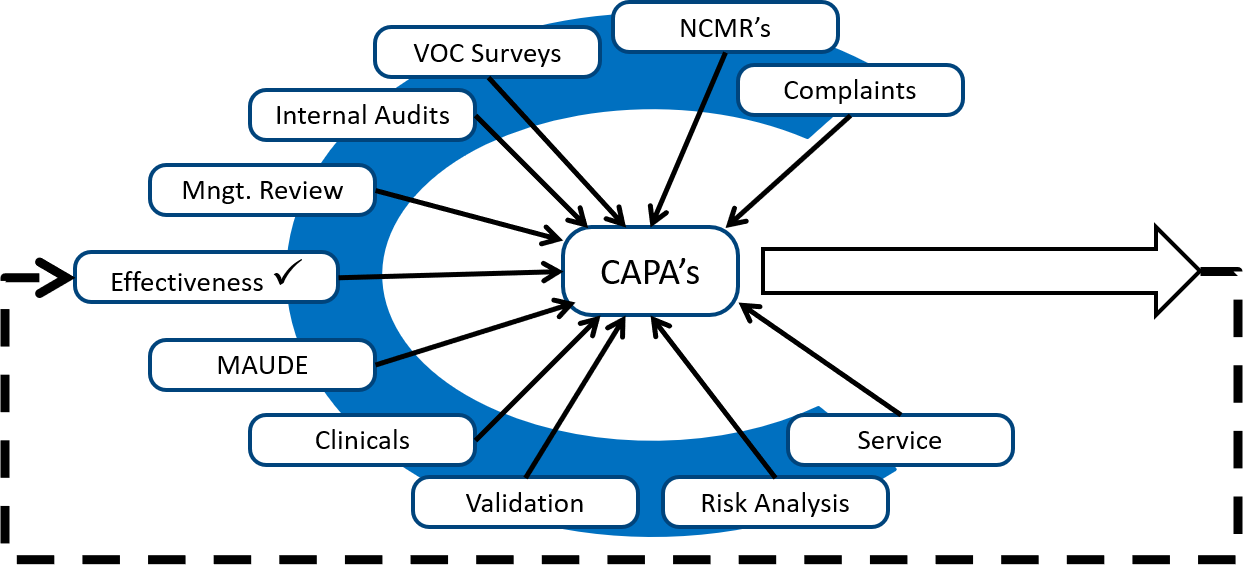
Guide A Comprehensive Understanding of CAPA
CAPA Training Riskbased
This Course Will Give You A Fundamental Understanding Of Corrective And Preventative Actions, Or Capa, In The Life Sciences.
Learn How To Implement And Maintain A Corrective And Preventive Action (Capa) System In Accordance With Ich Q10 And Cgmp Regulations.
You May Not Realize How Important Capa Can Be, Or In What Ways.
Elevating Perianesthesia Nursing Excellence, Knowledge, And Patient Advocacy Through Certification.
Related Post: