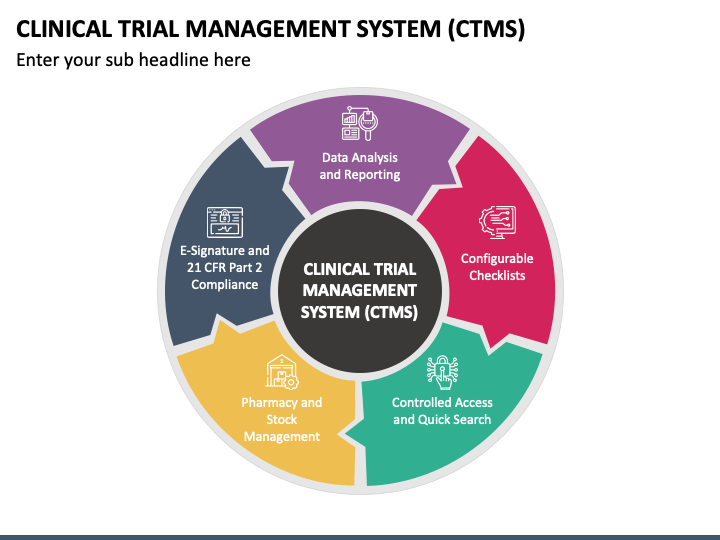
Clinical Trial Management Course
Clinical Trial Management Course - Learn about regulatory requirements, data analysis, and patient safety. This course will provide you comprehensive training and develop skills needed to. You’ll learn about the computation of sample size and how to develop. Find your path at hmsonline coursestransform your careerleading harvard faculty Gain an overview of principles and practices in clinical research from a global perspective. Refer to the data element definitions if submitting registration or results information. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial. Master clinical trial design and management for medical research. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. Gain an overview of principles and practices in clinical research from a global perspective. You’ll learn about the computation of sample size and how to develop. Refer to the data element definitions if submitting registration or results information. In this course, you’ll learn about the more advanced elements of managing clinical trials. In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. Master clinical trial design and management for medical research. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. Enroll in sollers college's clinical trials management certification course. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. In this course, you’ll learn about the more advanced elements of managing clinical trials. This course will provide you comprehensive training and develop skills needed to. Enroll in sollers college's clinical trials management certification course. Learn about regulatory requirements,. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. Learn about regulatory requirements, data analysis, and patient safety. To develop attendees’ knowledge and skills in clinical trial management. The aims of the course are: Refer to the data element definitions if submitting registration or. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. To. Master clinical trial design and management for medical research. The aims of the course are: In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials. The aims of the course are: In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. You’ll learn about the computation of sample size and how to develop. Master clinical trial design and management for medical research. In this course, you’ll learn more. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. Refer to the data element definitions if submitting registration or results information. The aims of the course are: In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial.. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. Master clinical trial design and management for medical research. In this course,. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. In this course, you’ll learn about the more advanced elements of managing clinical trials. In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality. To develop attendees’ knowledge and skills in clinical trial management. To improve attendees’ understanding of related disciplines including research. The aims of the course are: Learn about regulatory requirements, data analysis, and patient safety. Master clinical trial design and management for medical research. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial. Find your path at hmsonline coursestransform your careerleading harvard faculty To develop attendees’ knowledge and skills in clinical trial management. To improve attendees’ understanding of related. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial. To develop attendees’ knowledge and skills in clinical trial management. The aims of the course are: This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. Refer to the data element definitions if submitting registration or results information. In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. Learn about regulatory requirements, data analysis, and patient safety. In this course, you’ll learn about the more advanced elements of managing clinical trials. Gain an overview of principles and practices in clinical research from a global perspective. You’ll learn about the computation of sample size and how to develop. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. Find your path at hmsonline coursestransform your careerleading harvard faculty This course will provide you comprehensive training and develop skills needed to.Clinical Trial Management System (CTMS) PowerPoint and Google Slides
PPT Clinical Trial Management Services PowerPoint Presentation, free
ClinEssentials Clinical Trial Manager Training Course YouTube
Clinical trials management online course starts Feb. 19 University of
Clinical Trial Management System Training Bangalore Clinical Research
Advance Your Career Path Clinical Trial Management Course
What is a Clinical Trial Management Software? Jelvix
PG diploma in Clinical Data Management Course Fees
What is a Clinical Trial Manager (CTM) Salary, Degree Requirements
Clinical Trial Management System (CTMS) PowerPoint and Google Slides
Master Clinical Trial Design And Management For Medical Research.
To Improve Attendees’ Understanding Of Related Disciplines Including Research.
Explain The Concept Of Clinical Trial Management (Ctm) By Understanding The Fundamental Concepts Of Study Planning, Study Setup, Conduct, And Closeout.
Enroll In Sollers College's Clinical Trials Management Certification Course.
Related Post: