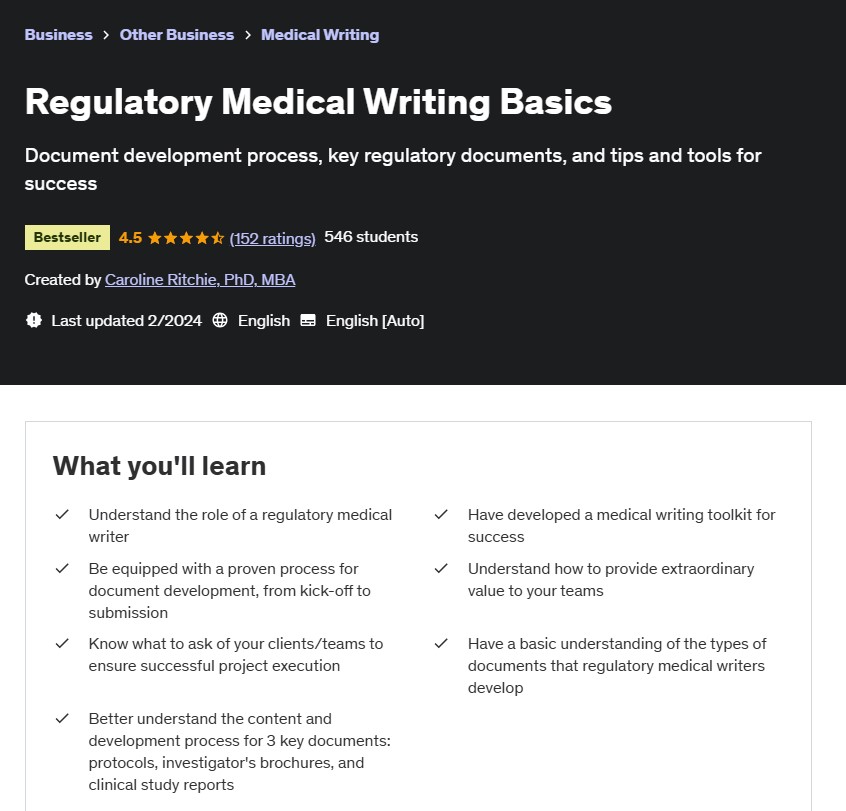
Regulatory Writing Courses
Regulatory Writing Courses - Our regulatory writing course is designed to master the art of writing accurate and compliant regulatory documents. Learn about health literacy, how to share clear research results, and more. The university of chicago’s certificate program in regulatory writing gives you the training you need to write submissions to the fda and other regulatory bodies. Invest in a online regulatory writing certificate from uchicago and train with experts at the top of their field; The certificate offers a mix of courses taught by experienced instructors working in the medical publishing field. Choose from dozens of flexible, online options in applied sciences, healthcare, and business. Check out the regulatory writing courses offered. The university of chicago’s certificate program in regulatory writing gives you the training to write submissions to the fda and other regulatory bodies. Scientific publications, cme, and other materials for healthcare professionals require specialized writing skills. Regulatory affairs & medical writing course provides a possibility to learn strategies, tricks, action plans to become confident in collaboration within a clinical and regulatory environment to create accurate, clearly worded documents. Our regulatory writing course equips professionals with the skills to write clear, accurate, and compliant documents for regulated industries. The university of chicago’s professional development certificate in regulatory writing gives you the training you need to write submissions to the fda and other regulatory bodies. Plain language is vital to communicating effectively. Our regulatory writing course is designed to master the art of writing accurate and compliant regulatory documents. Invest in a online regulatory writing certificate from uchicago and train with experts at the top of their field; Scientific publications, cme, and other materials for healthcare professionals require specialized writing skills. The university of chicago’s certificate program in regulatory writing gives you the training to write submissions to the fda and other regulatory bodies. Regulatory affairs & medical writing course provides a possibility to learn strategies, tricks, action plans to become confident in collaboration within a clinical and regulatory environment to create accurate, clearly worded documents. Engage in a program offering technical expertise along with soft skills; Learn with experts a possibility to learn strategies, tricks, action plans to become a confident regulatory writer. The university of chicago’s certificate program in regulatory writing gives you the training you need to write submissions to the fda and other regulatory bodies. Regulatory affairs & medical writing course provides a possibility to learn strategies, tricks, action plans to become confident in collaboration within a clinical and regulatory environment to create accurate, clearly worded documents. Our regulatory writing. Our online medical writing and editing certificate program is designed to teach you the core principles and best practices of crisp, clear, and sophisticated medical writing and editing. Scientific publications, cme, and other materials for healthcare professionals require specialized writing skills. After this course, you will. Learn how to structure reports, policies, and technical documentation that meet regulatory standards and. Choose from dozens of flexible, online options in applied sciences, healthcare, and business. This course, designed to introduce regulatory writing as both a broad and specific skill set, teaches the tools necessary to identify, edit, and contribute components of a biomedical regulatory packet. Participants learn how to present complex data clearly and concisely, and highlight implications with tact and balance.. And receive the advising, events, networking, and opportunities our unique certificate program offer. Our regulatory writing course equips professionals with the skills to write clear, accurate, and compliant documents for regulated industries. Check out the regulatory writing courses offered. The university of chicago’s certificate program in regulatory writing gives you the training you need to write submissions to the fda. Regulatory affairs & medical writing course provides a possibility to learn strategies, tricks, action plans to become confident in collaboration within a clinical and regulatory environment to create accurate, clearly worded documents. Our online medical writing and editing certificate program is designed to teach you the core principles and best practices of crisp, clear, and sophisticated medical writing and editing.. Plain language is vital to communicating effectively. Learn how to structure reports, policies, and technical documentation that meet regulatory standards and avoid costly errors. Uchicago's regulatory certificate covers the foundations of how to edit, write, and communicate medically. Tailored for a diverse audience, including writers, communicators, engineers, and it professionals, this course serves as a beacon, guiding participants toward the. Our online medical writing and editing certificate program is designed to teach you the core principles and best practices of crisp, clear, and sophisticated medical writing and editing. Students will work to develop the tools necessary to identify, edit, and contribute components of a biomedical regulatory packet. This course is designed to introduce regulatory writing as both a broad and. Our regulatory writing course equips professionals with the skills to write clear, accurate, and compliant documents for regulated industries. Our regulatory writing course is designed to master the art of writing accurate and compliant regulatory documents. Participants learn how to present complex data clearly and concisely, and highlight implications with tact and balance. Technical writing seamlessly blends the art of. Uchicago's regulatory certificate covers the foundations of how to edit, write, and communicate medically. Participants learn how to present complex data clearly and concisely, and highlight implications with tact and balance. Choose from dozens of flexible, online options in applied sciences, healthcare, and business. This advanced course will cover fda submissions, regulatory writing, supportive documentation for major clinical trial milestones,. The university of chicago’s certificate program in regulatory writing gives you the training you need to write submissions to the fda and other regulatory bodies. Our online medical writing and editing certificate program is designed to teach you the core principles and best practices of crisp, clear, and sophisticated medical writing and editing. Students will work to develop the tools. Our regulatory writing course equips professionals with the skills to write clear, accurate, and compliant documents for regulated industries. Tailored for a diverse audience, including writers, communicators, engineers, and it professionals, this course serves as a beacon, guiding participants toward the creation of effective technical documents tailored to specialized audiences. Regulatory affairs & medical writing course provides a possibility to learn strategies, tricks, action plans to become confident in collaboration within a clinical and regulatory environment to create accurate, clearly worded documents. This course is designed to introduce regulatory writing as both a broad and specific skill set. Invest in a online regulatory writing certificate from uchicago and train with experts at the top of their field; Our online medical writing and editing certificate program is designed to teach you the core principles and best practices of crisp, clear, and sophisticated medical writing and editing. Engage in a program offering technical expertise along with soft skills; Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. Uchicago's regulatory certificate covers the foundations of how to edit, write, and communicate medically. Dive into the future of healthcare communication with our regulatory writing certificate program at uchicago. And receive the advising, events, networking, and opportunities our unique certificate program offer. After this course, you will. The certificate offers a mix of courses taught by experienced instructors working in the medical publishing field. Check out the regulatory writing courses offered. The university of chicago’s certificate program in regulatory writing gives you the training to write submissions to the fda and other regulatory bodies. The university of chicago’s certificate program in regulatory writing gives you the training you need to write submissions to the fda and other regulatory bodies.Global Regulatory Writing & Consulting (GLOBAL) on LinkedIn Intro to
18 Medical Writing Courses To Take In 2025
Regulatory CMC Writing Royed Training
Regulatory CMC Writing Royed Training
PREVIEW CfPA's CMC Writing and Submission Strategies A Global
Medical Communicators’ Guide to Regulatory Writing
Mastering Regulatory Writing Skills Online
Regulatory Writing Course Available Onsite, Online and Virtual
Regulatory Writing an Overview, Second Edition by Lisa DeTora Goodreads
Regulatory Writer Training PDF
Technical Writing Seamlessly Blends The Art Of Communication With The Nuances Of Technology.
Find Regulatory Writing Topics With Amwa.
Choose From Dozens Of Flexible, Online Options In Applied Sciences, Healthcare, And Business.
Students Will Work To Develop The Tools Necessary To Identify, Edit, And Contribute Components Of A Biomedical Regulatory Packet.
Related Post: